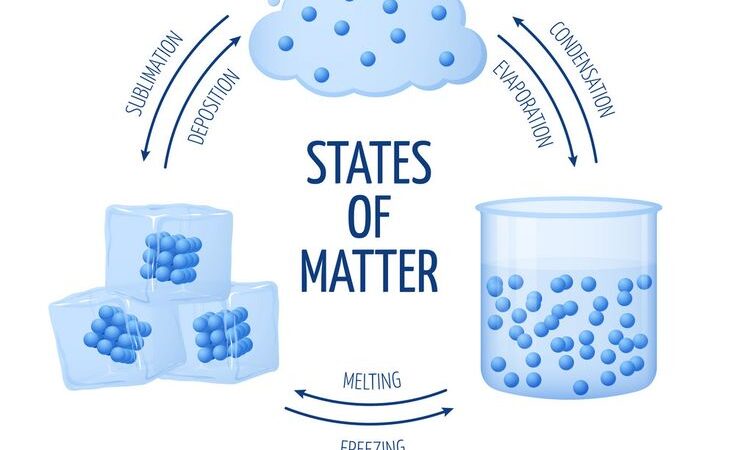
The states of matter refer to the distinct forms that different phases of matter take on. The four primary states of matter are solid, liquid, gas, and plasma. These states depend on the arrangement and behavior of atoms and molecules, which are influenced by temperature and pressure. Here’s a description of each state:
-
Solid:
- In solids, particles (atoms, molecules, or ions) are tightly packed together in a fixed arrangement.
- The particles vibrate but do not move from their fixed positions, leading to a definite shape and volume.
- Solids have a strong intermolecular force that prevents the particles from moving freely, which is why solids retain their shape and don’t flow.
-
Liquid:
- In liquids, particles are still close together but can move past one another.
- This allows liquids to flow and take the shape of their container while maintaining a constant volume.
- Liquids have a definite volume but no definite shape, meaning they can flow and adapt to the shape of the container.
-
Gas:
- In gases, particles are far apart and move freely and rapidly in all directions.
- Gases have neither a definite shape nor a definite volume. They expand to fill the shape and volume of their container.
- The intermolecular forces in gases are very weak, allowing the particles to spread out and move independently.
-
Plasma:
- Plasma is a high-energy state of matter where atoms are ionized, meaning electrons are stripped from atoms, creating positively charged ions and free electrons.
- It is found in stars, lightning, and certain high-energy laboratory conditions.
- Plasmas have properties distinct from solids, liquids, and gases, including high conductivity and sensitivity to magnetic fields.
Additionally, some materials can exist in other, less common states, such as Bose-Einstein condensates (BEC), which occur at extremely low temperatures where particles behave as a single quantum entity.
In summary, the states of matter are determined by the energy of the particles and how they interact with one another, and they can change with changes in temperature and pressure.
Topics of Course
-
STATES OF MATTER PART 1
16:34
-
STATES OF MATTER PART 2
12:27
-
STATES OF MATTER PART 3
16:36
-
STATES OF MATTER PART 4
22:09
-
STATES OF MATTER PART 5
33:30
-
STATES OF MATTER PART 6
20:52
-
STATES OF MATTER PART 7
22:24
-
STATES OF MATTER PART 8
11:35
-
STATES OF MATTER PART 9
10:24
-
STATES OF MATTER PART 10
16:53
-
STATES OF MATTER PART 11
27:30
-
STATES OF MATTER PART 12
26:00
-
STATES OF MATTER PART 13A Q&A
38:14
-
STATES OF MATTER PART 13B Q&A
29:48
-
STATES OF MATTER PART 14A EXAM STYLE QUESTIONS
33:09
-
STATES OF MATTER PART 14B EXAM STYLE QUESTIONS
35:13
Your Instructor
 Follow Me:
Follow Me:

 Follow Me:
Follow Me:

 Free
Free
